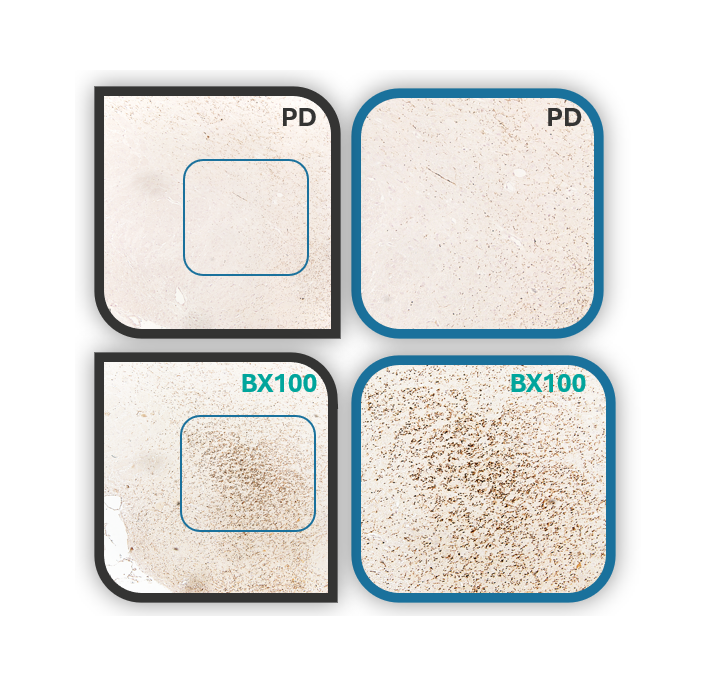
We Investigate the Role of EAAT2 Gene Pathways in Brain Neuroprotection
We are committed to altering the course of neurodegenerative diseases.
- In 2016, Chairman Vincent Chiang initiated a collaboration with Prof. Ying-Jui Ho from Chung Shan Medical University to develop a novel therapeutic candidate for Parkinson’s disease (PD), Parkinson’s disease dementia (PDD), and dementia with Lewy bodies (DLB). The project aims to address the underlying causes of these neurodegenerative disorders and to develop an innovative treatment capable of modifying disease progression.
- In 2017, an Investigational New Drug (IND) application was submitted to the U.S. FDA, enabling the launch of a multicenter, double-blind Phase II clinical trial in patients with PDD. The study progressed to the unblinding stage in the second quarter of 2025.